-
 Overview
Overview
Centre Testing International Group Co., Ltd. (CTI) is a market leader in testing, inspection, certification, calibration, audit, training & technical services; building trust between governments, enterprises, and consumers.
-
 Sustainability
Sustainability
By building a full value chain ESG governance system covering the strategic decision-making level, management execution level and business operation level, it actively practices penetrating management of ESG risk and opportunities, empowering sustainable development across the industry chain.
-
 Our service
Our serviceCentre Testing International Co., Ltd. (CTI) is the pioneer and leader in the TIC Industry which provides one-stop solutions on testing, inspection, certification, calibration, audit, training & technical services.
-
By Industry
Our service capabilties cover the upstream and downstream of the supply chain including textile and apparel,toys,electronic appliances,medical health,food...andother industries.
-
 Environment
Environment
-
 Raw Material & Fuel Chemicals
Raw Material & Fuel Chemicals
-
 Textiles, Apparel, Footwear & Accessories
Textiles, Apparel, Footwear & Accessories
-
 Food & Agricultural Products
Food & Agricultural Products
-
 Cosmetics, Personal Care & Household Chemicals
Cosmetics, Personal Care & Household Chemicals
-
 Building Materials&Construction Engineering
Building Materials&Construction Engineering
-
 Electronic & Electrical Appliances
Electronic & Electrical Appliances
-
 Toys, Furniture & Home Decoration
Toys, Furniture & Home Decoration
-
 Industrial Equipment & Manufacturing
Industrial Equipment & Manufacturing
-
 Rail & Aviation
Rail & Aviation
-
 Automotive & Spare Parts
Automotive & Spare Parts
-
 Pharma and Medical Services
Pharma and Medical Services
-
 Maritime Vessel Compliance Testing
Maritime Vessel Compliance Testing
 By Industry
By IndustryOur service capabilties cover the upstream and downstream of the supply chain including textile and apparel,toys,electronic appliances,medical health,food...andother industries.
-
-
 Specialty
SpecialtyComprehensively guarantee quality and safety, promote compliance and innovation, demonstrate brand competitiveness, and achieve higher quality, healthier, safer, and greener sustainable development.
-
 Management
ManagementWe have established a clear governance structure in accordance with listing requirements and national regulations and policies to deal with internal and external challenges and achieve sustainable development.
-
 Information DisclosureWe are committed to establishing normal and effective two-way communication with shareholders and investors. We have established a complete information disclosure mechanism to convey information to shareholders in a timely manner.
Information DisclosureWe are committed to establishing normal and effective two-way communication with shareholders and investors. We have established a complete information disclosure mechanism to convey information to shareholders in a timely manner.
-
 Talents Policy
Talents PolicyEnsuring the basic rights and benefits of employees;
Providing professional skills training to promote employees’ growth;
Carrying out various kinds of activities to balance employees’ work and life.
-
 RecruitmentWelcome to join CTI family! We are providing a platform for you to show your talents and achieve your career aspiration.
RecruitmentWelcome to join CTI family! We are providing a platform for you to show your talents and achieve your career aspiration.

QUALITY & VALUE
The API processes include chemical synthesis, semi-chemical synthesis, microbial fermentation, animal and plant extraction. Microbial fermentation is favored for its mild conditions and high yield. Controlling the process impurities of fermentation and animal and plant extraction has become an indispensable part of API quality research. CTI Pharma and Medical Services can provide you with DNA, protein and sugar residue detection in fermentation APIs.
?Service Introduction
Biological enzyme catalytic reactions have the advantages of high specificity, mild conditions, and green environmental protection, and are increasingly widely used in the production process of APIs. The International Enzyme Commission (I.E.C) divides enzymes into 6 major categories according to the type of reaction catalyzed by the enzyme - transferases, hydrolases, lyases, oxidoreductases, isomerases and ligases. The enzyme catalyst used to prepare APIs is a process impurity and its residue needs to be controlled.
Protein residue
The methods for determining protein content include Kjeldahl method, Folin phenol method (Lowry method), biuret method, BCA method and Coomassie brilliant blue method (Bradford method). Among them, Coomassie brilliant blue method is favored because of its highest sensitivity and low interference. This method can determine the amount of protein of 1~200ug.
DNA residues
CTI uses fluorescent staining to detect the residues of fermentation microbial DNA in raw materials.
Sugar residues
Whether it is starch, polysaccharides or oligosaccharides, the sugars can be converted into monosaccharides by hydrolysis, and the sugar residues can be detected by HPLC-CAD method.
?Service Background
Recently, CDE has also paid attention to the residues of enzymes, starch, DNA, etc. in the catalytic reaction when conducting new drug reviews for APIs synthesized by enzymes. CTI Pharma and Medical Services has established an analytical method for the residues of three components (DNA, protein, and sugar).
? Applicable products
Applicable to APIs prepared by enzyme catalysis or fermentation. Such as Vildagliptin, Demeclocycline hydrochloride, Atorvastatin calcium, Erythromycin, etc.
? Service content
Residue testing and method verification of three components (DNA, protein, sugar) in APIs.
According to the ChP 2020 Vol Ⅳ“9101 Guidelines for Validation of Analytical Method Adopted in Pharmaceutical Quality Specification” ,require for analytical methods and verification performance indicators, CTI performs method validation according to the requirements of the impurity quantitative analysis method.. The parameters examined include specificity, linearity/range, accuracy, precision (repeatability/intermediate precision), detection limit and quantification limit, and solution stability.
The sensitivity of the method can meet most application scenarios.
| Test items | Detection limit of the method | Limit of Quantitation |
| Protein residue | 0.6µg/mL | 2µg/mL |
| Sugar residue | 1.7µg/mL | 5µg/mL |
| DNA residue | 1.3ng/mL | 2.5ng/mL |
? Special services
For APIs that are easily soluble in water, the sample volume required for a single method test is only 2~3g, and 20~30g of sample is required for method development and validation.
? Service Process
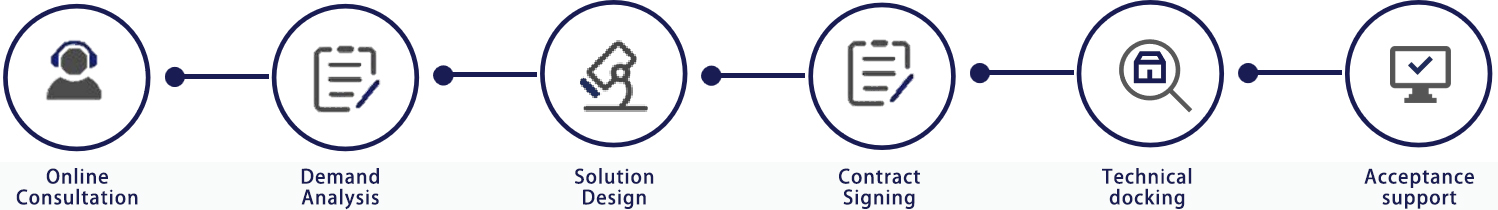























 粵公網(wǎng)安備 44030602000441號(hào)
粵公網(wǎng)安備 44030602000441號(hào)